Featured News - Current News - Archived News - News Categories
Don't Reuse Single-Use Dental Supplies
by mdsassociates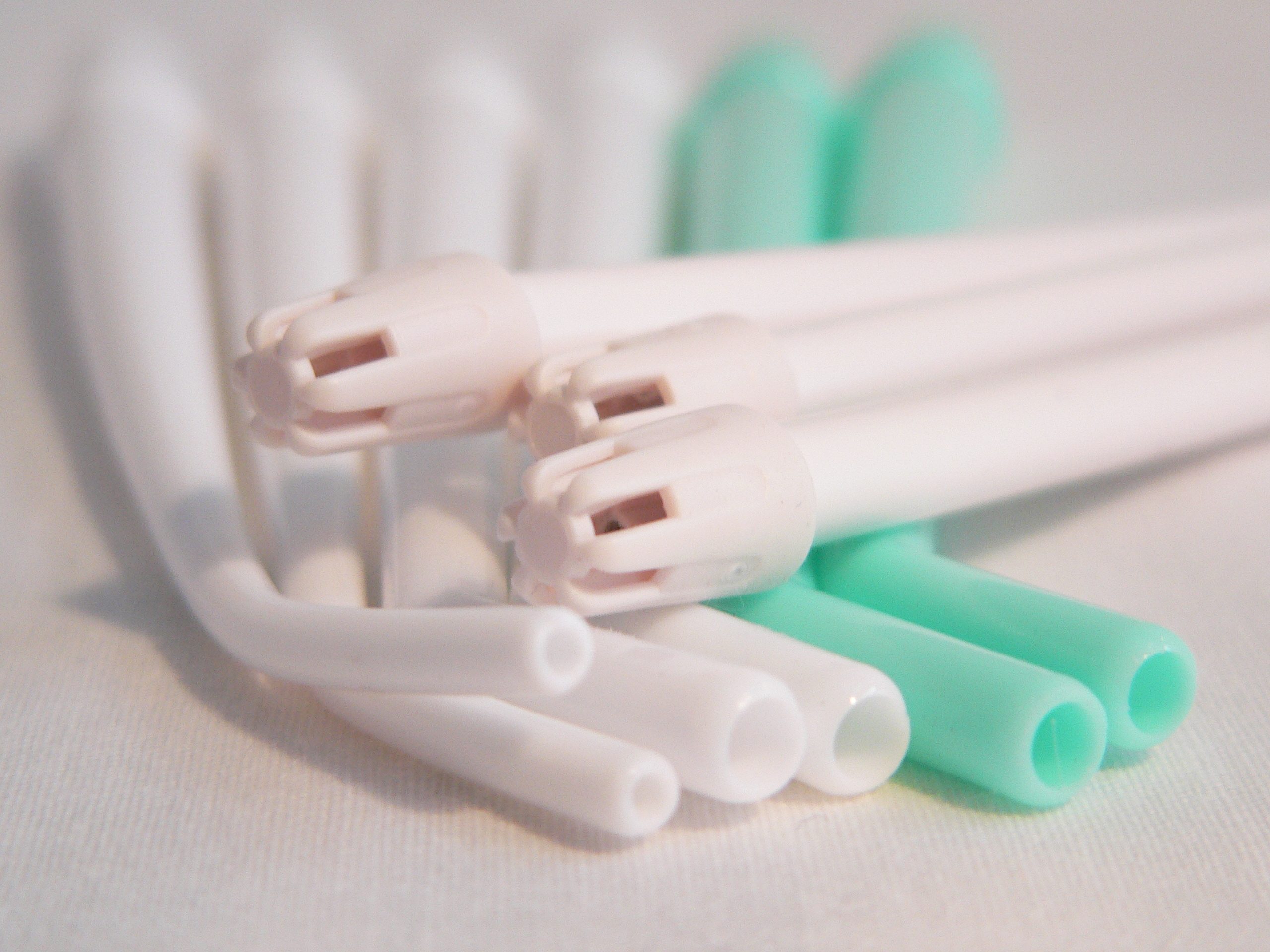
By definition, a disposable device is one that that is designed to be used on only one patient and then disposed of. The device may be one that is not heat tolerant, such as disposable mouth mirrors, prophy angles (DPA), plastic evacuation tips or air/water syringe tips. Since the device is designed for only one use, it is not tested for safety and efficacy for multiple uses and exposure to chemicals or heat during reprocessing and sterilization.
There is nothing that can justify knowingly putting patients at risk. Patient safety must always come first. If items cannot be adequately cleaned and sterilized for reuse, patients may be at risk of infectious disease transmission. Compromising patient safety to save money by reusing single-use items is an unacceptable practice.
The FDA regulations and CDC guidelines clearly preclude the reuse of disposable items. Yet the practice persists, primarily to save money on supply costs. Single-use or disposable devices cannot be adequately cleaned and sterilized for reuse and this puts patients at risk of infectious disease transmission.
For this reason, The U.S. Food and Drug Administration (FDA) has specific regulations regarding the labeling and reprocessing of single-use dental devices.
___________________________________________________________________________________________________